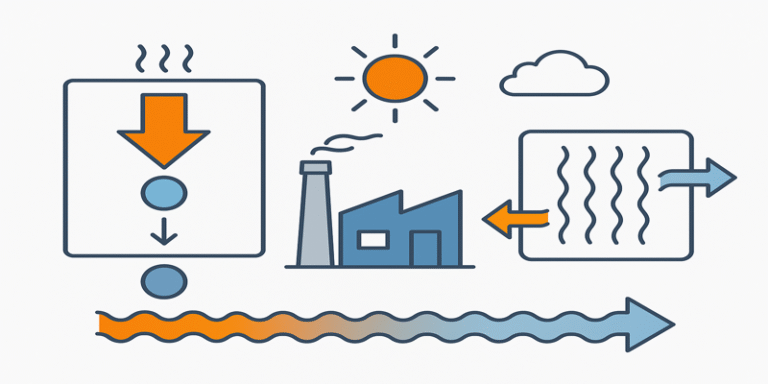
Thermodynamics and heat transfer are fundamental pillars of engineering, physics, and many applied sciences. While the two fields are closely related, they address different aspects of energy behaviour. Thermodynamics studies how energy is transferred and transformed into work, and heat transfer focuses on the mechanisms—conduction, convection, and radiation—by which thermal energy moves between systems.
Together, these principles underpin the design and operation of engines, refrigeration systems, power plants, and heating, ventilation, and air conditioning (HVAC) systems. In an era of climate change and growing concerns about energy efficiency, mastering these concepts is vital for engineers seeking to balance performance with sustainability (Çengel & Boles, 2015).
Thermodynamics: The Laws That Govern Energy
Thermodynamics deals with the relationships between heat, work, and energy in physical systems. Its principles are expressed through four main laws:
- Zeroth Law of Thermodynamics – Defines the concept of temperature and establishes that if two systems are each in thermal equilibrium with a third system, they are in thermal equilibrium with each other.
- First Law of Thermodynamics – States that energy cannot be created or destroyed, only converted from one form to another. This is essentially the law of conservation of energy.
- Second Law of Thermodynamics – Introduces the concept of entropy, stating that energy transformations are not 100% efficient and that systems naturally progress toward greater disorder.
- Third Law of Thermodynamics – States that as a system approaches absolute zero, the entropy approaches a minimum value.
These laws are universal—they apply equally to chemical reactions, mechanical systems, and even biological processes. For example, in power generation, the First Law helps track energy flows through a turbine, while the Second Law explains why waste heat must be released.
Heat Transfer: Mechanisms of Thermal Energy Flow
Heat transfer is concerned with how thermal energy moves. The three primary mechanisms are:
1.0 Conduction
Conduction occurs when heat flows through a material without the movement of the material itself. It relies on molecular collisions and electron movement. Metals such as copper and aluminium are excellent conductors due to their free electrons.
Example: The heating of a metal spoon when one end is placed in hot water is a result of conduction. In engineering, conduction analysis is crucial in designing heat exchangers and insulation materials (Incropera & DeWitt, 2007).
2.0 Convection
Convection involves the transfer of heat by the movement of fluids (liquids or gases). It can be:
- Natural convection, where fluid motion is driven by density differences due to temperature variations (e.g., warm air rising).
- Forced convection, where fluid motion is induced by external means such as fans or pumps.
Example: In HVAC systems, forced convection distributes warm or cool air efficiently throughout buildings.
3.0 Radiation
Radiation transfers energy through electromagnetic waves, without the need for a medium. All objects emit thermal radiation, with intensity depending on their temperature.
Example: The warmth felt from the Sun on your skin is due to radiant heat transfer through the vacuum of space.
Applications in Engineering and Industry
1.0 Engines
Internal combustion engines, steam turbines, and jet engines rely heavily on thermodynamic principles. Engineers use thermodynamic cycles—such as the Otto, Diesel, and Rankine cycles—to model and optimise performance.
For example, the Rankine cycle is the basis of most power plants, converting heat from fuel combustion or nuclear fission into mechanical work.
2.0 Refrigeration Systems
Refrigerators, freezers, and air conditioners operate on the vapour-compression cycle, which uses phase changes of refrigerants to absorb and reject heat. The Second Law of Thermodynamics dictates that work must be input to move heat from a cooler space to a warmer one.
3.0 Power Plants
Whether powered by coal, gas, nuclear, or renewable sources, power plants operate on thermodynamic cycles that involve heat transfer at multiple stages. Efficiency improvements in these systems are critical for reducing greenhouse gas emissions.
4.0 HVAC Systems
Heating, ventilation, and air conditioning rely on heat transfer mechanisms to maintain indoor comfort. Engineers optimise systems for energy efficiency, often integrating heat recovery units to reduce waste.
Energy Efficiency and Sustainability
In the context of climate change, thermodynamics plays a vital role in assessing and improving energy efficiency. Since the Second Law imposes limits on efficiency, engineers must focus on minimising losses and maximising the useful work extracted from energy inputs.
For example:
- Improving insulation reduces heat loss in buildings.
- Combined heat and power (CHP) systems capture waste heat from electricity generation for heating purposes.
- Regenerative braking in electric vehicles recovers kinetic energy.
Çengel and Ghajar (2020) stress that sustainable design increasingly requires integrating thermodynamic analysis into the early stages of system development.
Thermodynamics in Renewable Energy
Renewable energy systems also depend on thermodynamic principles:
- Solar thermal plants use mirrors to focus sunlight, heating a working fluid for power generation.
- Geothermal systems exploit natural heat from the Earth’s interior to produce electricity or direct heating.
- Wind turbines, though driven by aerodynamics, require thermodynamic analysis in their generators and cooling systems.
Advanced Topics and Research Trends
1.0 Heat Pipes
Heat pipes are highly efficient thermal conductors that use phase change and capillary action to transfer heat over long distances with minimal loss. They are increasingly used in electronics cooling and aerospace applications.
2.0 Nano-Scale Heat Transfer
With the miniaturisation of devices, understanding heat transfer at the nano-scale has become critical. At this scale, classical theories may not apply, requiring models that account for quantum effects.
3.0 Thermal Energy Storage
Energy storage solutions, such as molten salt systems in solar plants, rely on thermodynamic analysis to store heat for later use, improving renewable energy reliability.
4.0 Computational Fluid Dynamics (CFD)
CFD simulations allow engineers to model heat transfer in complex geometries, improving the design of heat exchangers, turbines, and cooling systems without costly experiments.
Educational and Professional Relevance
For engineering students, mastering heat transfer and thermodynamics builds a foundation for numerous specialisations, including mechanical engineering, chemical engineering, and aerospace engineering. Professional engineers apply these concepts daily to ensure that systems meet performance, safety, and sustainability goals.
Heat transfer and thermodynamics are inseparable fields that define how energy moves and transforms in our world. They provide the scientific basis for technologies that power our homes, transport us across the globe, preserve our food, and maintain our comfort.
In the 21st century, with the urgent need to combat climate change, these disciplines are more important than ever. By combining deep theoretical knowledge with innovative engineering solutions, we can design systems that are not only effective but also environmentally responsible.
References
Çengel, Y. A. & Boles, M. A. (2015). Thermodynamics: An Engineering Approach (8th ed.). McGraw-Hill Education.
Çengel, Y. A. & Ghajar, A. J. (2020). Heat and Mass Transfer: Fundamentals and Applications (6th ed.). McGraw-Hill Education.
Incropera, F. P. & DeWitt, D. P. (2007). Fundamentals of Heat and Mass Transfer (6th ed.). Wiley.